Pharmaceutical Tablets
Leading Manufacturers, Exporters, Wholesaler, Retailer, Distributor of Anamorelin 50mg Tablet, Anapolon 50mg Tablets, Crizalk 250 Mg Crizotinib Capsule, Enasidenib 50mg Tablet, Ketosteril Tablets, Lorbriqua 100 Mg Tablet, Lucibelzu Belzutifan Tablet40Mg, Lucicapiva Capivasertib Tablets200 Mg, Lucidabra Dabrafenib 75mg Capsules, Lucielace Elacestrant 345 Mg Tablet, Lucilorla Lorlatinib 100mg Tablets, Lucimito Mitotane Tablet 500mg, Lucitram (Trametinib) 2 Mg Tablet, Lucixaz Ixazomib 4mg, Lysodren Mitotane 500mg Tablet, Mekinist Trametinib 2mg Tablet, Scemblix Asciminib 40mg Tablet, Tafinlar Dabrafenib 75mg Capsules, Tagrisso 80 Mg Tablet, Tucatinib 150 Mg Tablet, Upadacitinib Rinvoq15Mg and Velpanat 400 Mg100mg Tablets from Bangalore.
| Business Type | Exporter, Supplier, Retailer |
| Packaging | Box |
| Color | White |
| Type | Anti Cancer Medicines |
| Form | Tablets |
| Packaging Size | 100 Tables in One Box |
| Material | Anamorelin Hydrochloride |
| Usage | Treat Cachexia In Cancer Patients |
| Dosage | 100mg Orally Once Daily Without Food |
| Place of Origin | India |
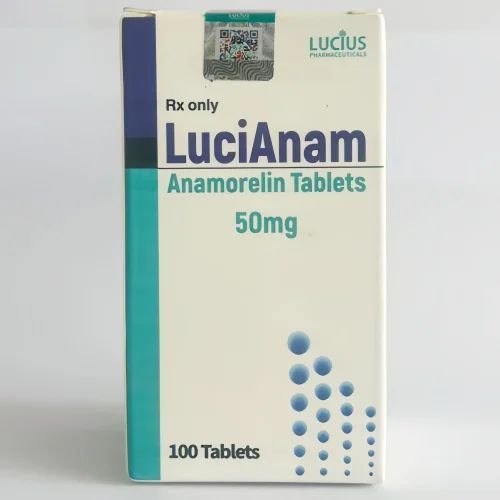
COMPOSITION:Each LuciAnam tablet contains Anamorelin hydrochloride. ……………………………. 50 mg
INDICATION:
LuciAnam is used to treat cachexia patients with unresectable advanced and recurrent non-small cell lung cancer, gastric cancer, pancreatic cancer and colorectal cancer. For cancer cachexia patients with poor efficacy such as nutritional therapy. For patients who have lost more than 5% of their weight within 6 months with loss of appetite and meet the following criteria:
① Fatigue or malaise,
② Decreased overall muscle strength,
③ Either a CRP value exceeding 0.5mg/dL, a hemoglobin value below 12g/dL, or an album in value below 3.2g/dL.
DOSAGE AND USE:
The recommended dosage for adults of LuciAnam is 100mg orally once daily without food.COMPOSITION:Each LuciAnam tablet contains Anamorelin hydrochloride. ……………………………. 50 mg
INDICATION:
LuciAnam is used to treat cachexia patients with unresectable advanced and recurrent non-small cell lung cancer, gastric cancer, pancreatic cancer and colorectal cancer. For cancer cachexia patients with poor efficacy such as nutritional therapy. For patients who have lost more than 5% of their weight within 6 months with loss of appetite and meet the following criteria:
① Fatigue or malaise,
② Decreased overall muscle strength,
③ Either a CRP value exceeding 0.5mg/dL, a hemoglobin value below 12g/dL, or an album in value below 3.2g/dL.
DOSAGE AND USE:
The recommended dosage for adults of LuciAnam is 100mg orally once daily without food.COMPOSITION:Each LuciAnam tablet contains Anamorelin hydrochloride. ……………………………. 50 mg
INDICATION:
LuciAnam is used to treat cachexia patients with unresectable advanced and recurrent non-small cell lung cancer, gastric cancer, pancreatic cancer and colorectal cancer. For cancer cachexia patients with poor efficacy such as nutritional therapy. For patients who have lost more than 5% of their weight within 6 months with loss of appetite and meet the following criteria:
① Fatigue or malaise,
② Decreased overall muscle strength,
③ Either a CRP value exceeding 0.5mg/dL, a hemoglobin value below 12g/dL, or an album in value below 3.2g/dL.
DOSAGE AND USE:
The recommended dosage for adults of LuciAnam is 100mg orally once daily without food.
| Business Type | Manufacturer, Exporter, Supplier, Retailer |
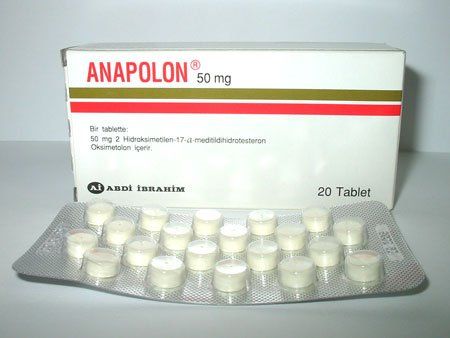
| Strength Per Tablet | 50 Mg |
| Tablet Quantity Per Strip | 20 |
| Product Name | Anapolon 50mg |
| Packaging Type | Box With Blister Strips |
| Color | White |
| Country of Origin | India |
| Active Ingredient | Oxymetholone |
| Dosage Form | Tablet |
| Product Code | Ana |
Available in a pack of 20 tablets in each strip.
| Business Type | Exporter, Supplier, Retailer |
| Storage | Store In A Cool, Dry Place |
| Side Effect | Nausea, Vomiting, Edema, Diarrhea |
| Dosage | As Prescribed By The Doctor |
| Country of Origin | Made In India |
| Form | Capsule |
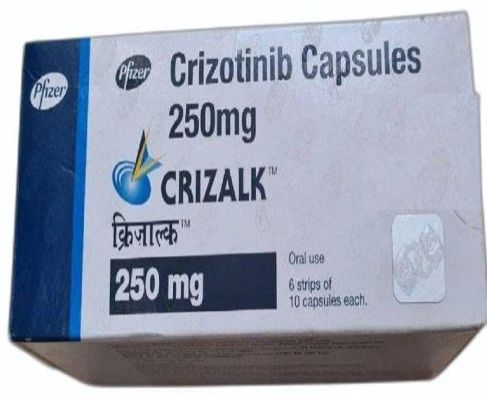
| Name | Crizalk 250 Mg Crizotinib Capsule |
| Type | Capsule |
| Usage | Treatment Of Non-small Cell Lung Cancer |
| Serious Side Effects | Severe Stomach Pain, Fever, Shortness Of Breath |
| Packaging Type | Bottle |
Crizalk 250mg Capsule is used in the treatment of non-small cell lung cancer that is locally advanced or has spread to other areas of the body.
Crizalk 250mg Capsule should be taken with food or without food, but try to have it at the same time every day to get the most benefits. Your doctor will decide what dose is necessary and how often you need to take it. This will depend on what you are being treated for and may change from time to time. You should take it exactly as your doctor has advised. Taking it in the wrong way or taking too much can cause very serious side effects. It may take several weeks or months for you to see or feel the benefits but do not stop taking it unless your doctor tells you to.
Nausea, vomiting, edema (swelling), and diarrhea are some common side effects of this medicine. Some serious side effects that might occur which need doctor consultation include severe stomach pain, fever, chills, shortness of breath, fast heartbeat, partial or complete loss of vision or changes in bowel habits. Your doctor may perform some blood tests during the treatment to check for liver function.Crizalk 250mg Capsule is used in the treatment of non-small cell lung cancer that is locally advanced or has spread to other areas of the body.
Crizalk 250mg Capsule should be taken with food or without food, but try to have it at the same time every day to get the most benefits. Your doctor will decide what dose is necessary and how often you need to take it. This will depend on what you are being treated for and may change from time to time. You should take it exactly as your doctor has advised. Taking it in the wrong way or taking too much can cause very serious side effects. It may take several weeks or months for you to see or feel the benefits but do not stop taking it unless your doctor tells you to.
Nausea, vomiting, edema (swelling), and diarrhea are some common side effects of this medicine. Some serious side effects that might occur which need doctor consultation include severe stomach pain, fever, chills, shortness of breath, fast heartbeat, partial or complete loss of vision or changes in bowel habits. Your doctor may perform some blood tests during the treatment to check for liver function.Crizalk 250mg Capsule is used in the treatment of non-small cell lung cancer that is locally advanced or has spread to other areas of the body.
Crizalk 250mg Capsule should be taken with food or without food, but try to have it at the same time every day to get the most benefits. Your doctor will decide what dose is necessary and how often you need to take it. This will depend on what you are being treated for and may change from time to time. You should take it exactly as your doctor has advised. Taking it in the wrong way or taking too much can cause very serious side effects. It may take several weeks or months for you to see or feel the benefits but do not stop taking it unless your doctor tells you to.
Nausea, vomiting, edema (swelling), and diarrhea are some common side effects of this medicine. Some serious side effects that might occur which need doctor consultation include severe stomach pain, fever, chills, shortness of breath, fast heartbeat, partial or complete loss of vision or changes in bowel habits. Your doctor may perform some blood tests during the treatment to check for liver function.
| Business Type | Exporter, Supplier, Retailer |
| Packaging | Box |
| Color | White |
| Composition | Enasidenib 50mg |
| Packaging Size | 30 Tablets in One Box |
| Indication | Treatment Of Adult Patients With Relapsed Or Refractory Acute Myeloid Leukemia (AML) With An Isocitrate Dehydrogenase-2 (IDH2) Mutation |
| Dosage | 100 Mg Taken Orally Once Daily With Or Without Food Until Disease Progression Or Unacceptable Toxicity |
| Formulation | Tablet |
| Usage | Oral Administration |
| Place of Origin | India |
COMPOSITION:
Each tablet contains: Enasidenib……… 50mg
INDICATION:
LuciEna is an isocitrate dehydrogenase-2 inhibitor indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation.
DOSAGE AND USE:
The recommended dosage of LuciEna is 100 mg taken orally once daily with or without food until disease progression or unacceptable toxicity.
Tablet should be swallowed whole & not chewed or crushed.COMPOSITION:
Each tablet contains: Enasidenib……… 50mg
INDICATION:
LuciEna is an isocitrate dehydrogenase-2 inhibitor indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation.
DOSAGE AND USE:
The recommended dosage of LuciEna is 100 mg taken orally once daily with or without food until disease progression or unacceptable toxicity.
Tablet should be swallowed whole & not chewed or crushed.
| Business Type | Exporter, Supplier, Retailer |
| Usage | Hospital,Clinical |
| Form | Tablets |
| Dosage | As prescribed by doctor |
| Storage | Cool & Dry Place |
| Packaging Type | Packed in Strips & Sachet |
| Purity | 100% |
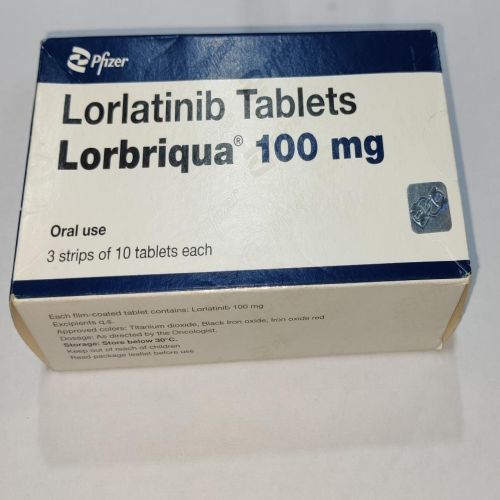
| Business Type | Exporter, Supplier, Retailer |
| Usage/Application | Treatment Of Metastatic NSCLC |
| Country of Origin | India |
| Packaging Size | 3 X 10 Tablets |
| Form | Tablet |
| Type | Lorbriqua Tablet |
| Active Ingredient | Lorlatinib |
| Drug Class | Tyrosine Kinase Inhibitor |
| Indicated For | Metastatic Non-Small Cell Lung Cancer (NSCLC) |
| Inactive Ingredient | Lactose |
| Potential Side Effects | Liver Damage, Decreased Fertility In Males |
| Monitoring Parameters | White Blood Cell Counts, Platelets, Blood Sugar, Electrolytes, Kidney Parameters |
| Precautions | Avoid Grapefruit Juice, Inform About Liver Disorders, Heart Failure, High Blood Cholesterol Levels, Or Hypertension |
| Allergy Warnings | Hypersensitive Reactions Like Itchy Skin Or Rashes |
Lorbriqua tablet is a drug belonging to the class of tyrosine kinase inhibitors, containing the active ingredient Lorlatinib. It is used to treat metastatic non-small cell lung cancer (NSCLC). This is the most common type of lung cancer. Metastatic non-small cell lung cancer (NSCLC) is a type of lung cancer that has spread to other parts of the body, such as the bones, liver, brain, or other organs.
During the treatment, your doctor may periodically monitor your white blood cell counts, platelets, blood sugar, electrolytes, and kidney parameters to prevent serious complications. This medicine has the potential to cause liver damage. Also, it may cause decreased fertility in males, which could affect your ability to father a child. Talk to your healthcare provider if you have concerns about fertility.
Inform to the doctor if you have been diagnosed with liver disorders, heart failure, high blood cholesterol levels, or hypertension before starting this treatment. Report to your doctor if you experience any hypersensitive reactions like itchy skin or rashes, while taking this medicine. It is advised to avoid drinking grapefruit juice while taking this drug, as it may increase the level of the drug in the blood and cause side effects.
This tablet contains lactose as an inactive ingredient, which the body is unable to digest. Lactose is a sugar found in milk and dairy products. It can cause symptoms such as bloating and abdominal pain. If you have lactose intolerance, inform your doctor before starting this therapy.Lorbriqua tablet is a drug belonging to the class of tyrosine kinase inhibitors, containing the active ingredient Lorlatinib. It is used to treat metastatic non-small cell lung cancer (NSCLC). This is the most common type of lung cancer. Metastatic non-small cell lung cancer (NSCLC) is a type of lung cancer that has spread to other parts of the body, such as the bones, liver, brain, or other organs.
During the treatment, your doctor may periodically monitor your white blood cell counts, platelets, blood sugar, electrolytes, and kidney parameters to prevent serious complications. This medicine has the potential to cause liver damage. Also, it may cause decreased fertility in males, which could affect your ability to father a child. Talk to your healthcare provider if you have concerns about fertility.
Inform to the doctor if you have been diagnosed with liver disorders, heart failure, high blood cholesterol levels, or hypertension before starting this treatment. Report to your doctor if you experience any hypersensitive reactions like itchy skin or rashes, while taking this medicine. It is advised to avoid drinking grapefruit juice while taking this drug, as it may increase the level of the drug in the blood and cause side effects.
This tablet contains lactose as an inactive ingredient, which the body is unable to digest. Lactose is a sugar found in milk and dairy products. It can cause symptoms such as bloating and abdominal pain. If you have lactose intolerance, inform your doctor before starting this therapy.
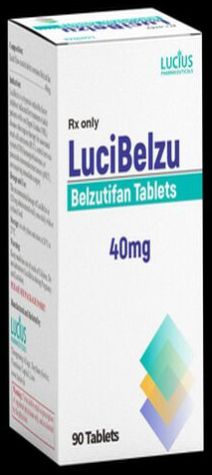
| Business Type | Exporter, Supplier, Retailer |
| Type | Anti Cancer Medicines |
| Form | Tablets |
| Packaging Size | 90 Tablets |
| Manufacturer | Lucibelzu Pharmaceuticals |
| Dosage | 40 Mg |
| Usage | Oral Consumption |
| Business Type | Exporter, Supplier, Retailer |
| Dosage | 200 Mg |
| Application | Treatment Of Adult Patients With Hormone Receptor-positive,HER2-negative,Locally Advanced Or Metastatic Breast Cancer With Specific Alterations |
| Packaging | Box |
| Storage | Store At Room Temperature |
| Type | Tablets |
| Packaging Size | 64 Tablets In One Box |
| Form | Tablet |
| Indication | Combination With Fulvestrant For Specified Breast Cancer Treatment |
| Route Of Administration | Oral |
LuciCapiva is a kinase inhibitor indicated, in combination with fulvestrant for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer with one or more PIK3CA/AKT1/PTEN-alterations as detected by an FDA-approved test following progression on at least one endocrine-based regimen in the metastatic setting or recurrence on or within 12 months of completing adjuvant therapy.
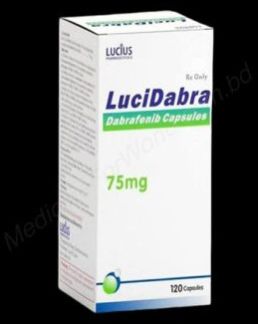
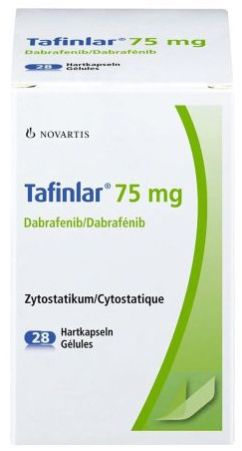
| Business Type | Exporter, Supplier, Retailer |
| Usage | Treatment Of Unresectable Or Metastatic Melanoma With BRAF V600E Mutation,Metastatic Non-small Cell Lung Cancer With BRAF V600E Mutation,Anaplastic Thyroid Cancer With BRAF V600E Mutation |
| Country of Origin | India |
| Packaging Size | 120 Capsules In Box |
| Form | Capsules |
| Color | White |
| Type | Tafinlar Dabrafenib Capsules |
| Material | Dabrafenib Mesylate |
| Indication | Kinase Inhibitor For Melanoma And Other Specified Cancers |
| Limitations Of Use | Not Indicated For Wild-type BRAF Melanoma, NSCLC, Or ATC |
COMPOSITION:
Each LuciDabra Capsule contains: 88.88mg Dabrafenib mesylate equivalent to Dabrafenib……………….. 75mg
INDICATION:
LuciDabra is a kinase inhibitor indicated as a single agent for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation.
LuciDabra is indicated, in combination with trametinib, for:
· the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations.
· the adjuvant treatment of patients with melanoma with BRAF V600E or V600K mutations and involvement of lymph node(s), following complete resection.
· the treatment of patients with metastatic non-small cell lung cancer (NSCLC) with BRAF V600E mutation.
· the treatment of patients with locally advanced or metastatic anaplastic thyroid cancer (ATC) with BRAF V600E mutation and with no satisfactory locoregional treatment options.
Limitations of Use: LuciDabra is not indicated for treatment of patients with wild-type BRAF melanoma, wild-type BRAF NSCLC, or wild-type BRAF ATC.COMPOSITION:
Each LuciDabra Capsule contains: 88.88mg Dabrafenib mesylate equivalent to Dabrafenib……………….. 75mg
INDICATION:
LuciDabra is a kinase inhibitor indicated as a single agent for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation.
LuciDabra is indicated, in combination with trametinib, for:
· the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations.
· the adjuvant treatment of patients with melanoma with BRAF V600E or V600K mutations and involvement of lymph node(s), following complete resection.
· the treatment of patients with metastatic non-small cell lung cancer (NSCLC) with BRAF V600E mutation.
· the treatment of patients with locally advanced or metastatic anaplastic thyroid cancer (ATC) with BRAF V600E mutation and with no satisfactory locoregional treatment options.
Limitations of Use: LuciDabra is not indicated for treatment of patients with wild-type BRAF melanoma, wild-type BRAF NSCLC, or wild-type BRAF ATC.COMPOSITION:
Each LuciDabra Capsule contains: 88.88mg Dabrafenib mesylate equivalent to Dabrafenib……………….. 75mg
INDICATION:
LuciDabra is a kinase inhibitor indicated as a single agent for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation.
LuciDabra is indicated, in combination with trametinib, for:
· the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations.
· the adjuvant treatment of patients with melanoma with BRAF V600E or V600K mutations and involvement of lymph node(s), following complete resection.
· the treatment of patients with metastatic non-small cell lung cancer (NSCLC) with BRAF V600E mutation.
· the treatment of patients with locally advanced or metastatic anaplastic thyroid cancer (ATC) with BRAF V600E mutation and with no satisfactory locoregional treatment options.
Limitations of Use: LuciDabra is not indicated for treatment of patients with wild-type BRAF melanoma, wild-type BRAF NSCLC, or wild-type BRAF ATC.
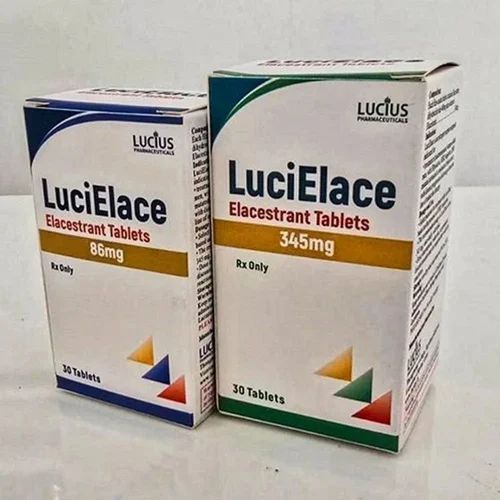
| Business Type | Exporter, Supplier, Retailer |
| Packaging | Box |
| Color | White |
| Type | Lucielace Elacestrant 345 Mg Tablet |
| Form | Tablets |
| Packaging Size | 30 Tablets in One Box |
| Usage | Treatment Of Estrogen Receptor (ER)-positive, HER2-negative Advanced Or Metastatic Breast Cancer |
| Dosage | 86 Mg Orally Once Daily |
| Administration | Take At The Same Time Each Day, Swallow Whole With Food |
| AdditionalInfo | Effective Even In Patients With ESR1 Mutations |
LuciElace 86 mg Tablet contains Elacestrant, a selective estrogen receptor degrader (SERD) indicated for the treatment of estrogen receptor (ER)-positive, HER2-negative advanced or metastatic breast cancer.
Uses:
✔ ER+/HER2- advanced or metastatic breast cancer
✔ Post-endocrine therapy resistance breast cancer
How LuciElace Works:
Elacestrant is a selective estrogen receptor degrader (SERD) that binds to the estrogen receptor (ER), leading to its degradation and inhibiting ER-driven tumor growth. It is effective even in patients with ESR1 mutations.
Dosage & Administration:
Recommended Dose: 86 mg orally once daily
Administration:
Take at the same time each day
Swallow whole with food; do not crush, chew, or split
Continue until disease progression or unacceptable toxicityLuciElace 86 mg Tablet contains Elacestrant, a selective estrogen receptor degrader (SERD) indicated for the treatment of estrogen receptor (ER)-positive, HER2-negative advanced or metastatic breast cancer.
Uses:
✔ ER+/HER2- advanced or metastatic breast cancer
✔ Post-endocrine therapy resistance breast cancer
How LuciElace Works:
Elacestrant is a selective estrogen receptor degrader (SERD) that binds to the estrogen receptor (ER), leading to its degradation and inhibiting ER-driven tumor growth. It is effective even in patients with ESR1 mutations.
Dosage & Administration:
Recommended Dose: 86 mg orally once daily
Administration:
Take at the same time each day
Swallow whole with food; do not crush, chew, or split
Continue until disease progression or unacceptable toxicity
| Business Type | Exporter, Supplier, Retailer |
| Application | Tablets Should Be Swallowed Whole & Not Chewed Or Crushed |
| Dosage | 100 Mg Orally Once Daily |
| Packaging Type | Box |
| Type | Lorlatinib Tablet |
| Country of Origin | India |
| Size | 30 Tablet In One Box |
| Form | Tablet |
| Color | White |
| Indication | Treatment Of Adult Patients With Metastatic Non-small Cell Lung Cancer (NSCLC) Whose Tumors Are ALK-positive |
EACH FILM-COATED LUCILORLA TABLET CONTAINS:
Lorlatinib……………………100mg
INDICATION:
LuciLorla is a kinase inhibitor indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors are anaplastic lymphoma kinase (ALK)-positive as detected by an FDA-approved test.
DOSAGE AND USE:
Recommended dosage: 100 mg orally once daily.
Severe Renal Impairment: 75 mg orally once daily.
Tablets should be swallowed whole & not chewed or crushed.EACH FILM-COATED LUCILORLA TABLET CONTAINS:
Lorlatinib……………………100mg
INDICATION:
LuciLorla is a kinase inhibitor indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors are anaplastic lymphoma kinase (ALK)-positive as detected by an FDA-approved test.
DOSAGE AND USE:
Recommended dosage: 100 mg orally once daily.
Severe Renal Impairment: 75 mg orally once daily.
Tablets should be swallowed whole & not chewed or crushed.
| Business Type | Exporter, Supplier, Retailer |
| Usage/Application | Swallow Whole, Do Not Chew Or Crush |
| Dosage | 2 G To 6 G Orally In Divided Doses Per Day |
| Country of Origin | India |
| Composition | Mitotane 500 Mg |
| Form | Tablet |
| Size | 100 Tablet in One Box |
| Type | 500mg Lucimito Mitotane Tablet |
| Color | White |
| Indication | Treatment Of Adrenal Cortical Carcinoma |
| Special Handling | Cytotoxic Drug, Follow Disposal Procedures |
COMPOSITION:
Each tablet contains Mitotane……………500 mg
INDICATION:
LUCIMITO is indicated for the treatment of patients with inoperable, functional or nonfunctional, adrenal cortical carcinoma.
DOSAGE AND USE:
The recommended initial dose of LUCIMITO is 2 g to 6 g orally, in three or four divided doses per day. Increase doses incrementally to achieve a blood concentration of 14 to 20 mg/L, or as tolerated. LUCIMITO is a cytotoxic drug. Follow applicable special handling and disposal procedures.
Tablets should be swallowed whole & not chewed or crushed.COMPOSITION:
Each tablet contains Mitotane……………500 mg
INDICATION:
LUCIMITO is indicated for the treatment of patients with inoperable, functional or nonfunctional, adrenal cortical carcinoma.
DOSAGE AND USE:
The recommended initial dose of LUCIMITO is 2 g to 6 g orally, in three or four divided doses per day. Increase doses incrementally to achieve a blood concentration of 14 to 20 mg/L, or as tolerated. LUCIMITO is a cytotoxic drug. Follow applicable special handling and disposal procedures.
Tablets should be swallowed whole & not chewed or crushed.
| Business Type | Exporter, Supplier, Retailer |
| Strength | 2 Mg |
| Packaging | Box |
| Color | White |
| Composition | Trametinib Dimethyl Sulfoxide |
| Packaging Size | 1 Blister Strip of 30 Tablets |
| Indication | Treatment Of Melanoma, Non-small Cell Lung Cancer, Anaplastic Thyroid Cancer |
| Formulation | Tablet |
| Usage | Single Agent Or In Combination With Dabrafenib |
| Place of Origin | India |
COMPOSITION:
Each Tablet contains: 2.254 mg Trametinib dimethyl sulfoxide equivalent to Trametinib……………… 2mg
INDICATION:
LuciTram is a kinase inhibitor indicated as a single agent for the treatment of BRAF-inhibitor treatment-naïve patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations.
LuciTram is indicated, in combination with dabrafenib, for:
· the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations.
· the adjuvant treatment of patients with melanoma with BRAF V600E or V600K mutations.
· the treatment of patients with metastatic non-small cell lung cancer (NSCLC) with BRAF V600E mutation.
· the treatment of patients with locally advanced or metastatic anaplastic thyroid cancer (ATC) with BRAF V600E mutation and with no satisfactory locoregional treatment options.COMPOSITION:
Each Tablet contains: 2.254 mg Trametinib dimethyl sulfoxide equivalent to Trametinib……………… 2mg
INDICATION:
LuciTram is a kinase inhibitor indicated as a single agent for the treatment of BRAF-inhibitor treatment-naïve patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations.
LuciTram is indicated, in combination with dabrafenib, for:
· the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations.
· the adjuvant treatment of patients with melanoma with BRAF V600E or V600K mutations.
· the treatment of patients with metastatic non-small cell lung cancer (NSCLC) with BRAF V600E mutation.
· the treatment of patients with locally advanced or metastatic anaplastic thyroid cancer (ATC) with BRAF V600E mutation and with no satisfactory locoregional treatment options.COMPOSITION:
Each Tablet contains: 2.254 mg Trametinib dimethyl sulfoxide equivalent to Trametinib……………… 2mg
INDICATION:
LuciTram is a kinase inhibitor indicated as a single agent for the treatment of BRAF-inhibitor treatment-naïve patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations.
LuciTram is indicated, in combination with dabrafenib, for:
· the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations.
· the adjuvant treatment of patients with melanoma with BRAF V600E or V600K mutations.
· the treatment of patients with metastatic non-small cell lung cancer (NSCLC) with BRAF V600E mutation.
· the treatment of patients with locally advanced or metastatic anaplastic thyroid cancer (ATC) with BRAF V600E mutation and with no satisfactory locoregional treatment options.
| Business Type | Exporter, Supplier, Retailer |
| Use | Proteasome Inhibitor For Multiple Myeloma Treatment |
| Packaging Size | 3 X 1 Tablets |
| Packaging Type | Box |
| Form | Capsules |
| Type | Capsule |
| Dosage Instructions | 4 Mg Orally On Days 1, 8, And 15 Of A 28-day Cycle |
| Administration | Oral |
| Food Interaction | Take At Least One Hour Before Or At Least Two Hours After Food |
| Treatment | Cancer |
| Country of Origin | India |
COMPOSITION:
Each LuciXaz capsule contains: 5.7mg lxazomib citrate equivalent to lxazomib….………4mg
INDICATION:
LuciXaz is a proteasome inhibitor indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy.
DOSAGE AND USE:
Recommended starting dose of 4 mg taken orally on Days 1, 8, and 15 of a 28-day cycle. Dose should be taken at least one hour before or at least two hours after food.
Capsule should be swallowed whole & not chewed or crushed.COMPOSITION:
Each LuciXaz capsule contains: 5.7mg lxazomib citrate equivalent to lxazomib….………4mg
INDICATION:
LuciXaz is a proteasome inhibitor indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy.
DOSAGE AND USE:
Recommended starting dose of 4 mg taken orally on Days 1, 8, and 15 of a 28-day cycle. Dose should be taken at least one hour before or at least two hours after food.
Capsule should be swallowed whole & not chewed or crushed.COMPOSITION:
Each LuciXaz capsule contains: 5.7mg lxazomib citrate equivalent to lxazomib….………4mg
INDICATION:
LuciXaz is a proteasome inhibitor indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy.
DOSAGE AND USE:
Recommended starting dose of 4 mg taken orally on Days 1, 8, and 15 of a 28-day cycle. Dose should be taken at least one hour before or at least two hours after food.
Capsule should be swallowed whole & not chewed or crushed.
| Business Type | Exporter, Supplier, Retailer |
| Color | White |
| Type | Mitotane Tablet |
| Application | Treat Cancer Of The Adrenal Glands By Slowing The Growth Of Adrenal Gland Cells And Decreasing Hormone Production. |
| Form | Tablet |
| Packaging | Box |
| Country of Origin | India |
| Size | 100 Tablet in One Box |
| Dosage | Take Orally With Fat-containing Food 3-4 Times Daily. |
Uses
Mitotane is used to treat cancer of the adrenal glands. It works by slowing the growth of or killing adrenal gland cells and also decreases the amount of hormones made by the adrenal gland.
How to use Lysodren
Read the Medication Guide and, if available, the Patient Information Leaflet provided by your pharmacist before you start taking mitotane and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth with fat-containing food as directed by your doctor, usually 3 or 4 times daily. The dosage is based on your medical condition and response to treatment.
Swallow the tablets whole. Do not crush, chew or break the tablets. Do not take tablets that are broken or crushed.Uses
Mitotane is used to treat cancer of the adrenal glands. It works by slowing the growth of or killing adrenal gland cells and also decreases the amount of hormones made by the adrenal gland.
How to use Lysodren
Read the Medication Guide and, if available, the Patient Information Leaflet provided by your pharmacist before you start taking mitotane and each time you get a refill. If you have any questions, ask your doctor or pharmacist.
Take this medication by mouth with fat-containing food as directed by your doctor, usually 3 or 4 times daily. The dosage is based on your medical condition and response to treatment.
Swallow the tablets whole. Do not crush, chew or break the tablets. Do not take tablets that are broken or crushed.
| Business Type | Exporter, Supplier, Retailer |
| Packaging | Strip |
| Color | White |
| Type | Film Coated Tablet |
| Form | Tablets |
| Dosage | 2mg |
| Usage | Treatment Of Melanoma, Thyroid Cancer, And Non-small Cell Lung Cancer |
| Combination | May Be Used In Combination With Dabrafenib |
| Place of Origin | India |
Mekinist Trametinib 2 mg Film Coated Tablets may be used alone or in combination with another medication(dabrafenib) to treat a type of skin cancer (melanoma). It is also used with dabrafenib to treat thyroid cancer and a type of lung cancer (non-small cell lung cancer-NSCLC). Trametinib works by slowing the growth of cancer cells.
| Business Type | Exporter, Supplier, Retailer |
| Packaging | Bottle |
| Dosage Form | 40 Mg |
| Color | White |
| Type | Anti Cancer Medicine |
| Form | Tablets |
| Packaging Size | 60 Tablets in One Bottle |
| Usage | Treatment Of Philadelphia Chromosome-positive Chronic Myeloid Leukemia (PH+ CML) In Adults |
| Place of Origin | India |
Scemblix (asciminib) is commonly used for treating certain kinds of Philadelphia chromosome-positive chronic myeloid leukemia (PH+ CML) in adults. Chronic myeloid leukemia (CML) is a type of blood cancer. The Philadelphia chromosome occurs when pieces of 2 chromosomes, chromosomes 9 and 22, break off and swap places. The new abnormal chromosome that is created is known as Philadelphia chromosome-positive (PH+). The PH+ affects your bone marrow and changes your white blood cells, causing leukemia. Scemblix may be used to treat certain PH+ CML.
How Scemblix Works for Chronic Myeloid Leukemia
Scemblix may also be used for other conditions as determined by your healthcare provider.Scemblix (asciminib) is commonly used for treating certain kinds of Philadelphia chromosome-positive chronic myeloid leukemia (PH+ CML) in adults. Chronic myeloid leukemia (CML) is a type of blood cancer. The Philadelphia chromosome occurs when pieces of 2 chromosomes, chromosomes 9 and 22, break off and swap places. The new abnormal chromosome that is created is known as Philadelphia chromosome-positive (PH+). The PH+ affects your bone marrow and changes your white blood cells, causing leukemia. Scemblix may be used to treat certain PH+ CML.
How Scemblix Works for Chronic Myeloid Leukemia
Scemblix may also be used for other conditions as determined by your healthcare provider.Scemblix (asciminib) is commonly used for treating certain kinds of Philadelphia chromosome-positive chronic myeloid leukemia (PH+ CML) in adults. Chronic myeloid leukemia (CML) is a type of blood cancer. The Philadelphia chromosome occurs when pieces of 2 chromosomes, chromosomes 9 and 22, break off and swap places. The new abnormal chromosome that is created is known as Philadelphia chromosome-positive (PH+). The PH+ affects your bone marrow and changes your white blood cells, causing leukemia. Scemblix may be used to treat certain PH+ CML.
How Scemblix Works for Chronic Myeloid Leukemia
Scemblix may also be used for other conditions as determined by your healthcare provider.
| Business Type | Exporter, Supplier, Retailer |
| Packaging | Bottle Or Blister Pack |
| Color | White |
| Type | Capsules |
| Storage | Store In A Cool, Dry Place |
| Usage | Treatment Of Melanoma And Non-small Cell Lung Cancer With BRAF V600 Mutation |
| Dosage | 75mg |
| Active Substance | Dabrafenib |
| Indicated For | Melanoma (skin Cancer) And Non-small Cell Lung Cancer |
| Additional Information | Used Alone Or In Combination With Trametinib |
| Place of Origin | India |
Tafinlar is a cancer medicine used to treat adults whose cancer cells have a specific genetic mutation (change) called ‘BRAF V600’. It is used for the treatment of:
melanoma (a skin cancer) that has spread or cannot be removed surgically. Tafinlar is used on its own or in combination with another cancer medicine, trametinib;
advanced (stage III) melanoma after surgery for it. Tafinlar is used in combination with trametinib;
advanced non-small cell lung cancer. It is used in combination with trametinib.
Tafinlar contains the active substance dabrafenib.Tafinlar is a cancer medicine used to treat adults whose cancer cells have a specific genetic mutation (change) called ‘BRAF V600’. It is used for the treatment of:
melanoma (a skin cancer) that has spread or cannot be removed surgically. Tafinlar is used on its own or in combination with another cancer medicine, trametinib;
advanced (stage III) melanoma after surgery for it. Tafinlar is used in combination with trametinib;
advanced non-small cell lung cancer. It is used in combination with trametinib.
Tafinlar contains the active substance dabrafenib.Tafinlar is a cancer medicine used to treat adults whose cancer cells have a specific genetic mutation (change) called ‘BRAF V600’. It is used for the treatment of:
melanoma (a skin cancer) that has spread or cannot be removed surgically. Tafinlar is used on its own or in combination with another cancer medicine, trametinib;
advanced (stage III) melanoma after surgery for it. Tafinlar is used in combination with trametinib;
advanced non-small cell lung cancer. It is used in combination with trametinib.
Tafinlar contains the active substance dabrafenib.
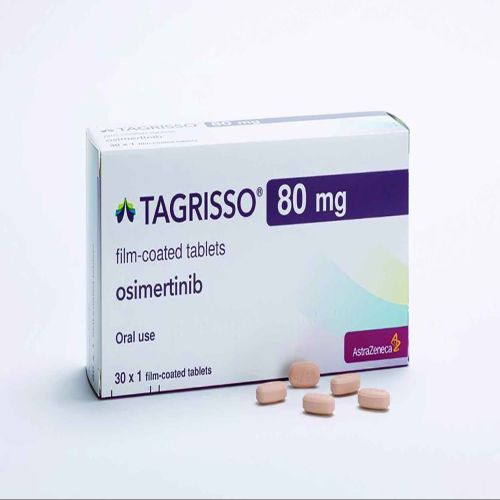
| Business Type | Exporter, Supplier, Retailer |
| Packaging Type | Typical Blister Packaging |
| Side Effects | Common Side Effect Of Diarrhea, May Cause Decreased White Blood Cells |
| Application | Treatment Of Non-small Cell Lung Cancer With Certain EGFR Mutations |
| Type | Tablet |
| Country of Origin | Made In India |
| Packaging Size | 1 X 30 Tablets |
| Form | Tablet |
| Color | White |
| Material | Protein Kinase Inhibitor |
| Dosage Instructions | Take With Or Without Food At The Same Time Daily As Prescribed By The Doctor |
| Storage Instructions | Store In A Cool, Dry Place Away From Direct Sunlight |
Tagrisso 80mg Tablet is a protein kinase inhibitor used in the treatment of non-small cell lung cancer. It is used in the treatment of adult patients who have certain estimated glomerular filtration rate (EGFR) mutations.
Tagrisso 80mg Tablet can be taken with or without food, but try to have it at the same time every day to get the most benefits. Your doctor will decide what dose is necessary and how often you need to take it. This will depend on what you are being treated for and may change from time to time. You should take it exactly as your doctor has advised. Taking it in the wrong way or taking too much can cause very serious side effects. It may take several weeks or months for you to see or feel the benefits but do not stop taking it unless your doctor tells you to.
Diarrhea is very common side effect of this medicine, so drink plenty of fluids. But, you must inform your doctor if it does not stop. You must have to inform your doctor if you experience difficulty in breathing along with fever and cough, or severe peeling of the skin. This medicine may reduce the number of blood cells (decrease white blood cells) in your blood, thereby, increasing the susceptibility to infections. Regular blood tests are required to check your blood cells.Tagrisso 80mg Tablet is a protein kinase inhibitor used in the treatment of non-small cell lung cancer. It is used in the treatment of adult patients who have certain estimated glomerular filtration rate (EGFR) mutations.
Tagrisso 80mg Tablet can be taken with or without food, but try to have it at the same time every day to get the most benefits. Your doctor will decide what dose is necessary and how often you need to take it. This will depend on what you are being treated for and may change from time to time. You should take it exactly as your doctor has advised. Taking it in the wrong way or taking too much can cause very serious side effects. It may take several weeks or months for you to see or feel the benefits but do not stop taking it unless your doctor tells you to.
Diarrhea is very common side effect of this medicine, so drink plenty of fluids. But, you must inform your doctor if it does not stop. You must have to inform your doctor if you experience difficulty in breathing along with fever and cough, or severe peeling of the skin. This medicine may reduce the number of blood cells (decrease white blood cells) in your blood, thereby, increasing the susceptibility to infections. Regular blood tests are required to check your blood cells.Tagrisso 80mg Tablet is a protein kinase inhibitor used in the treatment of non-small cell lung cancer. It is used in the treatment of adult patients who have certain estimated glomerular filtration rate (EGFR) mutations.
Tagrisso 80mg Tablet can be taken with or without food, but try to have it at the same time every day to get the most benefits. Your doctor will decide what dose is necessary and how often you need to take it. This will depend on what you are being treated for and may change from time to time. You should take it exactly as your doctor has advised. Taking it in the wrong way or taking too much can cause very serious side effects. It may take several weeks or months for you to see or feel the benefits but do not stop taking it unless your doctor tells you to.
Diarrhea is very common side effect of this medicine, so drink plenty of fluids. But, you must inform your doctor if it does not stop. You must have to inform your doctor if you experience difficulty in breathing along with fever and cough, or severe peeling of the skin. This medicine may reduce the number of blood cells (decrease white blood cells) in your blood, thereby, increasing the susceptibility to infections. Regular blood tests are required to check your blood cells.
| Business Type | Exporter, Supplier, Retailer |
| Packaging | Box |
| Color | White |
| Type | Anti Cancer Medicines |
| Form | Tablets |
| Packaging Size | 60 Tablets in One Box |
| Usage | Treatment Of Certain Types Of HER2-positive Breast Cancer |
| Dosage | 150 Mg |
| Targeted Therapy | Yes |
| Primary Use | Treatment Of Advanced Or Metastatic Breast Cancer |
| Additional Info | Selective HER2 Tyrosine Kinase Inhibitor |
LuciTuca 150mg contains Tucatinib, a targeted therapy used primarily in the treatment of certain types of HER2-positive breast cancer. It represents a significant advancement in oncology, particularly in managing advanced or metastatic breast cancer that has spread beyond the breast, including to the brain. Tucatinib, developed as a selective HER2 tyrosine kinase inhibitor, is a valuable tool in the evolving landscape of personalized cancer therapy
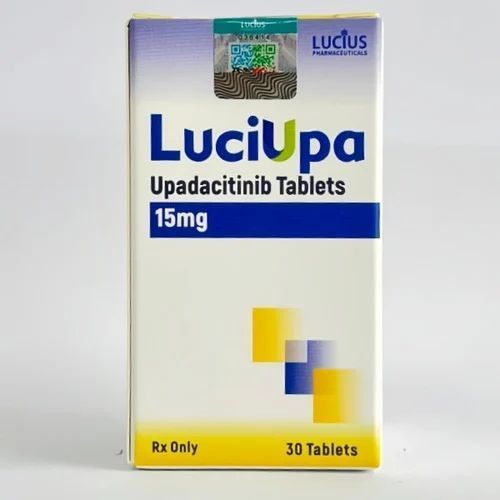
| Business Type | Exporter, Supplier, Retailer |
| Type | Medication |
| Dosage | 15 Mg |
Upadacitinib is used to treat moderate to severely active rheumatoid arthritis, active psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis (a type of arthritis in the spine) with objective signs of swelling, moderate to severe ulcerative colitis, moderate to severe Crohn 's disease, and polyarticular juvenile idiopathic arthritis (PJIA) in patients who have taken other medicines (eg, methotrexate) that did not work well. It is also used to treat moderate to severe atopic dermatitis (eczema) in patients who have taken other medicines that did not work well and whose condition is not well controlled with other treatments or in patients who cannot tolerate these treatments. This medicine is also used to treat giant cell arteritis.
Upadacitinib is a Janus kinase (JAK) inhibitor that works on the immune system.
This medicine is available only with your doctor 's prescription.Upadacitinib is used to treat moderate to severely active rheumatoid arthritis, active psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis (a type of arthritis in the spine) with objective signs of swelling, moderate to severe ulcerative colitis, moderate to severe Crohn 's disease, and polyarticular juvenile idiopathic arthritis (PJIA) in patients who have taken other medicines (eg, methotrexate) that did not work well. It is also used to treat moderate to severe atopic dermatitis (eczema) in patients who have taken other medicines that did not work well and whose condition is not well controlled with other treatments or in patients who cannot tolerate these treatments. This medicine is also used to treat giant cell arteritis.
Upadacitinib is a Janus kinase (JAK) inhibitor that works on the immune system.
This medicine is available only with your doctor 's prescription.
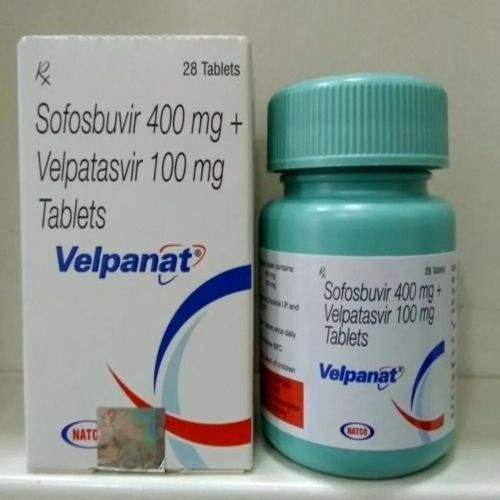
| Business Type | Exporter, Supplier, Retailer |
| Usage/Application | Treatment Of Chronic Hepatitis C Virus (HCV) Infection |
| Packaging Type | Bottle |
| Country of Origin | Made In India |
| Packaging Size | 28 Tablet In One Bottle |
| Form | Tablet |
| Type | Velpanat 400 Mg100mg Tablets |
| Dosage | Prescribed Dose And Duration, With Or Without Food |
| Side Effects | Fatigue, Anemia, Nausea, Headache, Insomnia, Diarrhea |
| Precautions | Inform Doctor About Health Conditions, Avoid Alcohol Consumption, Complete Course |
Product introduction
Velpanat Tablet is a combination of two antiviral medicines. This prescription medicine is used in the treatment of chronic hepatitis C virus (HCV) infection. It fights against the viruses to resolve the infection.
Velpanat Tablet should be taken in the prescribed dose and duration. It can be taken with or without food, but take it at the same time daily. It is advised not to consume more than the recommended dose. It is important to inform your doctor if you have any health conditions such as liver or kidney disease. It is harmful to consume alcohol along with this medicine, so it is advised to limit or avoid alcohol. The course of the medicine should be completed for better results.
The common side effects of this medicine include fatigue, anemia, nausea, headache, insomnia, and diarrhea. You should drink plenty of fluid and eat a healthy diet to prevent or overcome the side effects. Before taking the medicine, inform your doctor if you are taking any other medicines or supplementsProduct introduction
Velpanat Tablet is a combination of two antiviral medicines. This prescription medicine is used in the treatment of chronic hepatitis C virus (HCV) infection. It fights against the viruses to resolve the infection.
Velpanat Tablet should be taken in the prescribed dose and duration. It can be taken with or without food, but take it at the same time daily. It is advised not to consume more than the recommended dose. It is important to inform your doctor if you have any health conditions such as liver or kidney disease. It is harmful to consume alcohol along with this medicine, so it is advised to limit or avoid alcohol. The course of the medicine should be completed for better results.
The common side effects of this medicine include fatigue, anemia, nausea, headache, insomnia, and diarrhea. You should drink plenty of fluid and eat a healthy diet to prevent or overcome the side effects. Before taking the medicine, inform your doctor if you are taking any other medicines or supplements